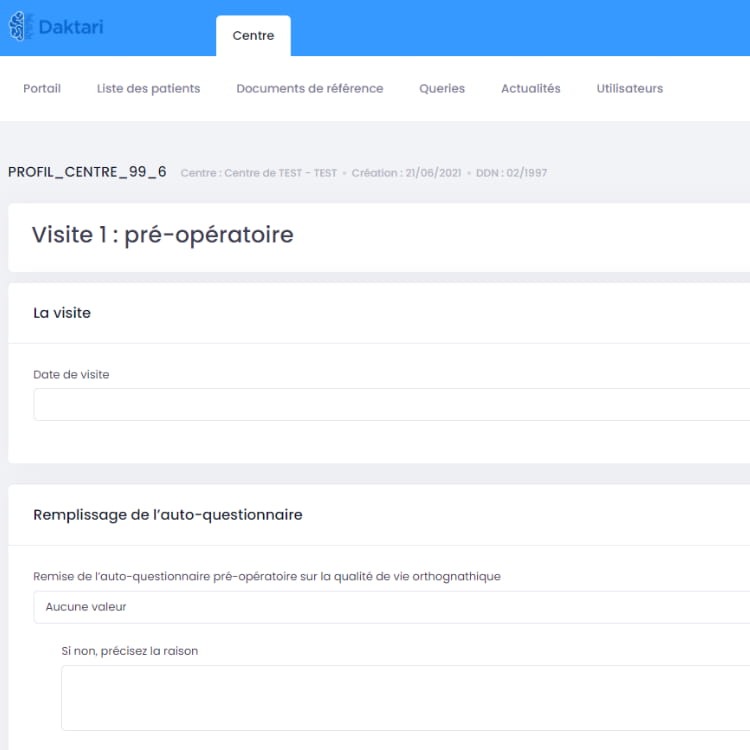
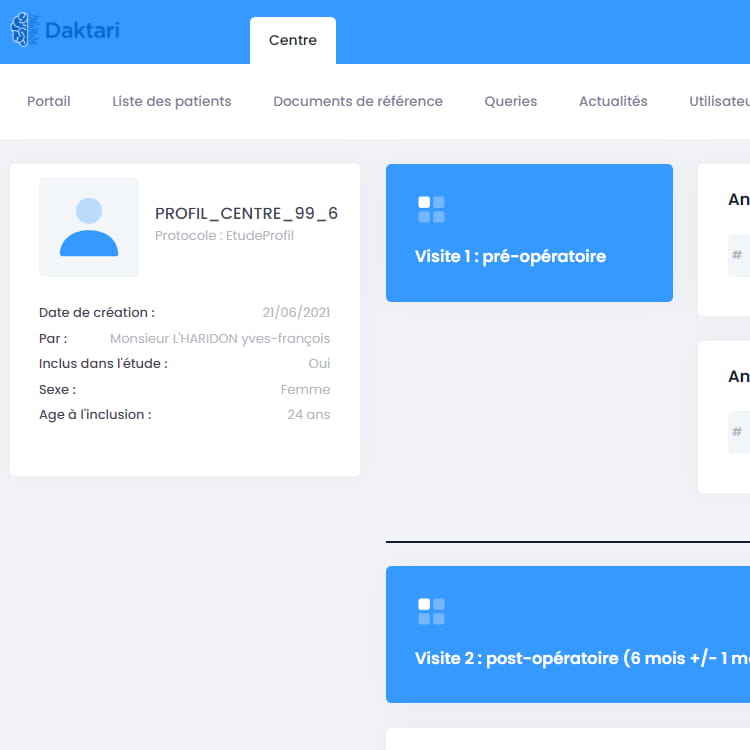
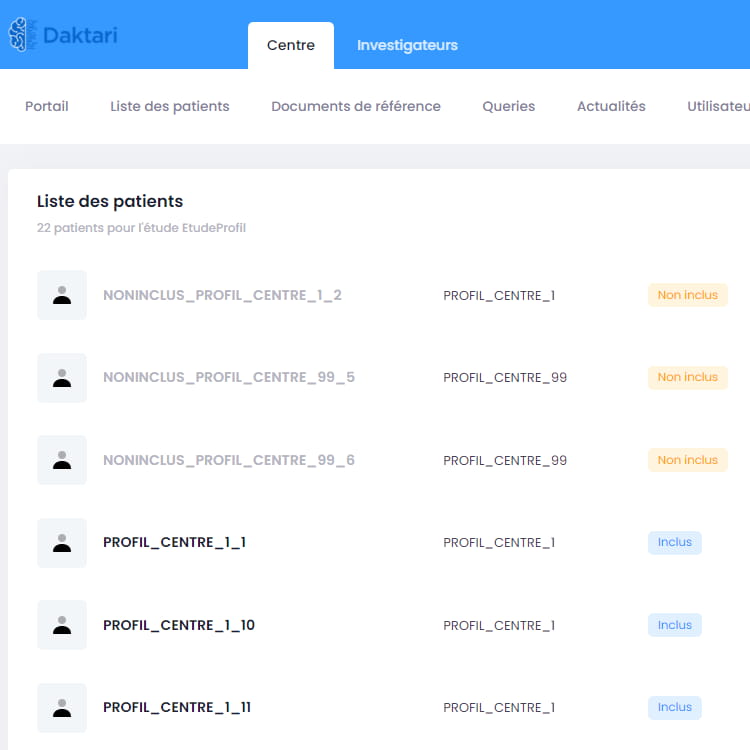
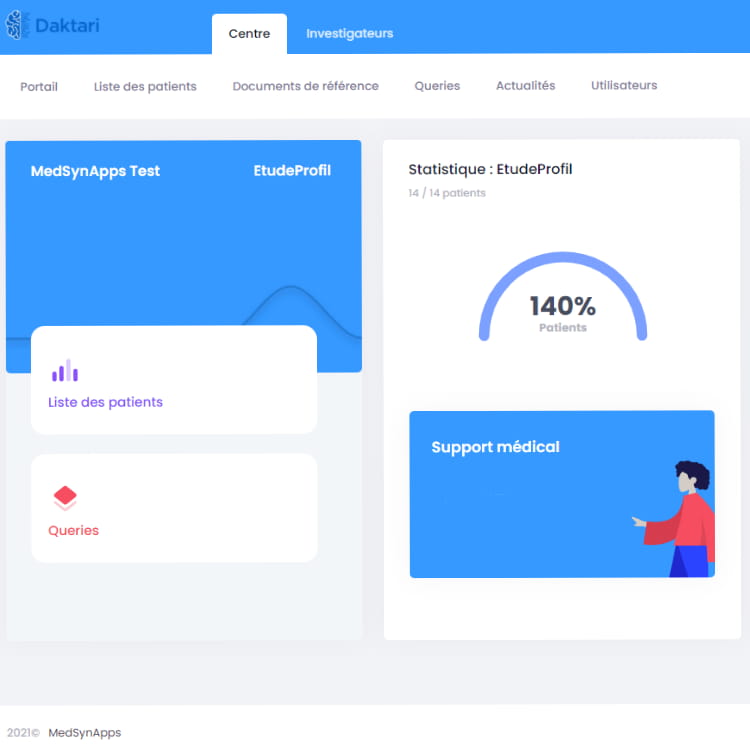
Comprehensive CTMS solutions allow for successful and secure clinical trials through authentic relationships and rich trial lifecycle analytics.
Launching a clinical trial requires dozens of moving parts to be successful, and managing those parts can be a challenge, even for the most experienced company. That’s where Daktari comes in.
Role-based workflows, reusable templates and automated reporting tools make it easy for all key participants in the trial review process to explore trends and outliers. Detect hidden data, safety and efficacy issues, medical monitoring, data integrity validation and statistical analyses.
Web-based software helps to accelerate patient randomization processes by clustering and applying algorithms that can help to ensure treatment group balance. We can help you with developing software that can tackle randomization, blind management and clinical supply administration.
iOT devices like wearables, smartphones, and in-home health devices enable precise patient monitoring and enhance the flexibility of clinical trials. iOT devices equip companies-sponsors, investigators, and subjects with innovative insights and analytics that can drastically change the way we receive healthcare. The data obtained from iOT devices helps life science companies to make better decisions and gain a competitive advantage.